Search All Blogs By Keyword
URGENT: The Hidden Full Spectrum CBD Ban & How to Protect Your Wellness Supply
🕒 4 min readIf you use CBD for stress, sleep, or pain relief, you need to read this immediately. A dangerous, confusing rule passed by the U.S. Senate […]
A Science-Backed Guide to CBD for Pain, Mood, and Sleep
🕒 5 min readFor millions, chronic pain is more than a physical sensation—it’s a constant battle that affects sleep, mood, and overall quality of life. While many have […]
A Comprehensive Guide to CBD for Anxiety: The Science & Evidence
🕒 4 min readFor the millions of people navigating the daily challenges of anxiety, the search for effective, natural support is a constant journey. In the last decade, […]
A Comprehensive Guide to Using CBD for Sleep
🕒 4 min readAs our world becomes increasingly demanding, quality sleep has become a luxury many can’t afford. Sleep disorders like insomnia affect millions, leading to a decline […]
Beyond CBD: 3 Pillars of Healthy Aging for a Vibrant Life
🕒 3 min readWhat is the real secret to a long, happy, and vibrant life? After spending time in a retirement community, I saw the answer wasn’t found […]
CBD for Healthy Aging: A Guide to Supporting Vitality
🕒 3 min readAfter spending a month visiting my parents in their retirement community, one thing became incredibly clear: age is just a number, but vitality is a […]
Exploring CBD for Parkinson’s Symptom Support
🕒 2 min readLiving with Parkinson’s disease presents daily challenges, from managing motor symptoms to navigating the emotional and mental impact of the condition. While there is no […]
Supporting Cognitive Health: A Guide to CBD, Memory, and Brain Wellness
🕒 3 min readAs we and our loved ones age, maintaining cognitive health becomes a primary focus. The journey can be challenging, filled with questions about how to […]
Supporting Resilience: CBD and the Body’s Response to Traumatic Stress
🕒 2 min readNavigating life after a traumatic event is a profound challenge that requires immense strength and a robust support system. For those seeking holistic tools to […]
Exploring CBD for Mood & Mental Wellness | A Science-Based Look
🕒 2 min readIn the search for balance and well-being, many are turning to natural options to support their mental health. One of the most talked-about is Cannabidiol […]
From Opiates to Relief: A Customer’s Journey with Whole Flower CBD
🕒 3 min readFrom Opiates to Relief: A Customer’s Journey with Whole Flower CBD There really is hope! The story below is from a client who was suffering […]
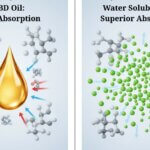
CBD Oil – Why Water Soluble Fluid is Achieving Much Better Results
🕒 2 min readThe “CBD Oil” Misconception We’ve noticed that in Europe and the UK, “CBD oil” is often used as a catch-all term. Many people, especially in […]
CBD for Bone Health: How It Helps Heal Fractures
🕒 2 min readFor years, the wellness community has known about CBD’s benefits for pain and stress, but one of its most remarkable applications lies in bone health. […]
Is CBD FDA-Approved? The Truth About Epidiolex & The Legal Status of CBD in 2025
🕒 3 min readIt’s one of the most common questions from new and experienced CBD users alike: Is CBD actually approved by the FDA? The answer is a […]
The Senior’s Guide to CBD: How It Helps Pain, Sleep & Anxiety in 2025
🕒 4 min readThe idea that cannabis is only for the young is fading fast. In fact, one of the most significant wellness trends is happening among older […]
CBD for Epilepsy: Understanding the Science and FDA Approval
🕒 3 min readFor families navigating the devastating impact of severe epilepsy, hope often comes from unexpected places. The story of CBD and epilepsy is one such powerful […]
A Guide to CBD for Athletes & Recovery
🕒 3 min readEvery athlete, from the weekend warrior to the seasoned professional, knows the cycle: you push your body to its limits, and then you manage the […]
The Endocannabinoid System Explained: How CBD Works
🕒 4 min readHow can one plant compound, cannabidiol (CBD), help with so many different aspects of our well-being—from mood and sleep to pain and inflammation? The answer […]
Does CBD Turn Into THC in Your Stomach? The Scientific Answer
🕒 2 min readIt’s a question we see online, often rooted in outdated studies: can the CBD you take orally convert into psychoactive THC in your stomach? It’s […]
CBD for Multiple Sclerosis (MS): More Than Symptom Relief?
🕒 2 min readLiving with Multiple Sclerosis (MS) means navigating a complex and often unpredictable journey. For years, the focus of many treatments has been on managing symptoms. […]
A Guide to CBD for Pets: Supporting Your Animal’s Wellness
🕒 3 min readFew things are more rewarding than seeing an older pet rediscover their youthful spark. We love hearing stories from our customers about how, after just […]
Cannabis vs. Alcohol: A 2025 Look at a Flawed Double Standard
🕒 2 min readFor generations, a strange paradox has lived at the heart of our culture. We’ve been taught to fear the supposed dangers of cannabis while culturally […]
Hemp Soil Decontamination: A Powerful Environmental Benefit
🕒 3 min readOne of the most profound and lesser-known benefits of the humble hemp plant is its ability to cleanse the very earth it grows in. Through […]
The Future of Wellness: Our New Article on CBD and AI
🕒 < 1 min readThe world of natural wellness is constantly evolving, and at The CBD Expert, we are always looking toward the future. One of the most exciting […]